
Unveiling India’s Drug Safety Crisis
Have you ever wondered how safe the medications we trust really are? The shocking Coldrif tragedy in Madhya Pradesh, where at least 24 infant deaths were linked to contaminated cough syrup, has thrown India’s drug safety system into the spotlight. This catastrophe beckons urgent questions about how well our government and regulators protect the public. As Dr. Raghuram Rajan poignantly remarked, “Safety in drug regulation is not an option; it’s a moral imperative that defines our society.” It raised an immediate and urgent question: if a simple syrup can be fatal, how trustworthy is India’s drug safety system? This wasn’t just a sudden news flash, but a painful wake-up call for parents, doctors, and regulators nationwide. History shows these drug safety lapses aren’t isolated incidents, and the health of our children should never be left to chance. In this blog, I take you through the dark corridors of India’s drug safety challenges, historic failures, regulatory lapses, and what must be done to ensure that such tragedies don’t occur in future.
The Sequence of Events: From Illness to Investigation
At first, local doctors thought the infant deaths were just a sad coincidence. However, when more children showed similar symptoms, red flags went up across Shivpuri and Sheopur. The response was reasonably quick – authorities took action by suspending Coldrif’s suspect batches and sounded the alarm with the Central Drugs Standard Control Organisation (CDSCO). The state health department joined in, racing to trace and quarantine all suspected medicines.
Forensic labs started checking the samples, and soon dangerous toxins were detected. But here’s the thing – despite news reports saying tests proved contamination, lab officials sometimes couldn’t agree on details, demanding more samples or clearer results. Media coverage turned the issue national, with people throughout India demanding better answers and accountability. All that confusion didn’t help anxious families who were desperate for the truth about what happened to their children.
Root Cause: What Went Wrong?
Lab findings pointed to diethylene glycol (DEG) and ethylene glycol – two industrial chemicals infamous for being deadly in children’s medicines. These kinds of chemicals end up in drugs when industry suppliers take shortcuts, like using industrial-grade solvents instead of pharmaceutical ones, often to save money. Sometimes, safety checks are skipped, and manufacturers push out new batches without proper verification. Whenever corners are cut, patients pay the price.
What’s most frustrating is that this isn’t just a new problem. From 2000 onwards, there have been at least eight major cases across India involving DEG or similarly hazardous contaminants nationwide. Too often, shortcuts, weak monitoring, and profit motives overshadow patient safety. Each time, you’d hope lessons would be learned, but often the same tragic mistakes repeat. Frankly, this is tough to accept as a responsible citizen. It’s a pattern that exposes deep flaws in India’s drug safety system. Even after decades, the same toxins keep reappearing. Why haven’t we learned from past mistakes?
Major Drug Safety Tragedies in India (2000–2025)
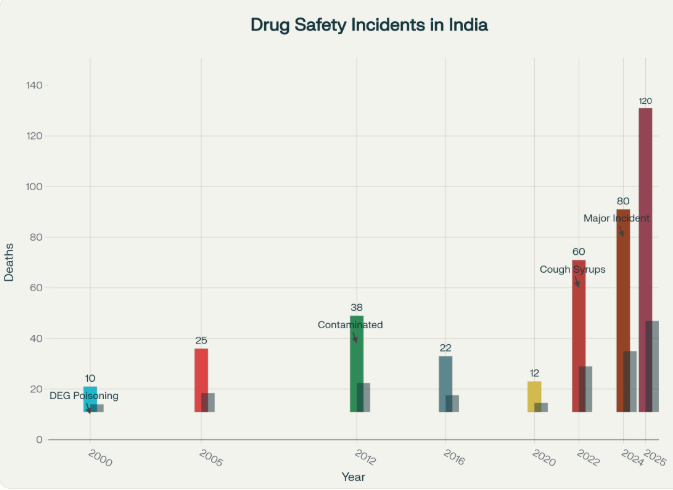
Source: National Health Ministry, PharmacyPro.io, Government Safety Reports
Regulatory Gaps Exposed: India’s Drug Safety System Under Scrutiny
India’s drug safety system is riddled with cracks that allow tragedies like the Coldrif one to unfold. The regulatory framework is fragmented, with multiple authorities like CDSCO and state-level regulators often duplicating roles or worse, neglecting enforcement. Unlike a single centralized watchdog, fragmented oversight fosters delays and negligence. The World Health Organization flagged these gaps recently, stating that India needs “streamlined and stringent regulatory reforms to ensure public health safety.” Many public testing labs suffer from lack of funding, staff shortages, and outdated equipment. Sadly, corruption and weak enforcement further erode the system’s effectiveness. Without a unified mechanism, contaminated drugs slip through and reach vulnerable populations, causing fatal outcomes.
The grim numbers speak volumes: in 2025, over 3,000 drug batches failed quality tests, with 245 spurious drug cases and 72 government bans issued (Source: PharmacyPro.io). Yet, penalties remain often insufficient to deter repeat offenders. Supply chains are complex and difficult to fully monitor, and patient safety is compromised. Lack of transparent, fast recall systems means contaminated products can linger on shelves longer and reach patients unnoticed. This fragmented setup weakens the India drug safety system’s ability to handle the growing pharmaceutical market effectively. While laws exist, their enforcement reflects mixed results. I believe it’s clear that stronger, more unified oversight is needed to prevent such future tragedies.
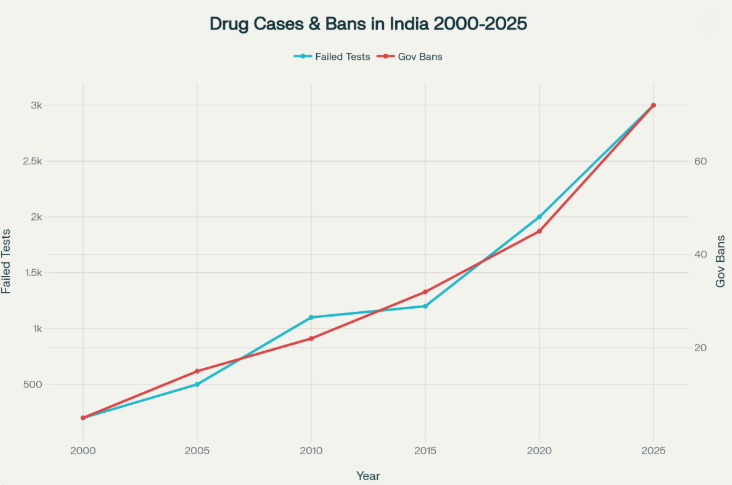
Source: National Health Ministry, PharmacyPro.io, WHO, Government Data
Lessons from Past Tragedies: Have We Learned Enough?
This tragedy is not new in India’s pharmacovigilance history. In 1986, Mumbai witnessed a glycerol syrup contamination that killed 14 while 11 died in Bihar in 1988, in a similar incident. Since then, repeated instances of unregulated medicines have endangered lives. The recurring pattern underlines how little progress has been made. From 2000 through 2025, multiple high-profile drug poisoning incidents claimed dozens of lives, especially among children. The 1998 DEG tragedy in Gurugram (33 child deaths), and the 2020 deaths in J&K due to contaminated cough syrup (17 child deaths) have all underscored the same systematic failures. Each event brought promises of change, yet meaningful action proved elusive. Another troubling pattern is the presence of the same chemical contaminants – DEG and ethylene glycol – showing a grave lack of oversight.
Globally, too, Indian syrups have triggered international recalls – in Gambia, Uzbekistan, and more – showing this is not just a national concern. The World Health Organization and India’s own public health experts have repeatedly flagged gaps in surveillance and accountability. Dr. Mira Shiva once remarked, “History keeps sending the same message. Our willingness to learn, however, must be stronger than our failures.” But outdated labs, lax checks, and diluted responsibility let history repeat itself. Almost every time, there’s a government inquiry or task force, but changes tend to be slow and enforcement uneven. Despite widespread condemnation, even basic reforms, such as mandatory reporting, have moved slowly.
The Way Forward: Systemic Reforms India Urgently Needs
So, what can actually help? For starters, every batch of medicine should go through independent safety tests before reaching any pharmacy. Offenders cutting corners must face severe, public consequences. We badly need more investment in labs, inspectors, and technology like QR codes and blockchain to trace every medicine. If recall data and test reports were totally open to everyone, more parents and doctors could keep watchful eyes. A centralized, online portal must publish batch test results and recall notices, giving citizens and doctors immediate access to crucial data. India should implement whistleblower protections, enabling insiders to flag concerns without fear.
Globally, the US FDA and EU EMA already run much more transparent and fast-moving recall systems. India’s not lacking smart people; we just need smart systems and the will to enforce them. Periodic third-party audits, public awareness campaigns, and regular government briefings can keep the public and press engaged. Technology exists for robust, transparent oversight; our systems must match our ambition for safety. It honestly feels like all the right tools are there, but someone needs to connect the dots and make it standard practice.
Empowering the Public: What Citizens Should Know
Even as officials work on fixes, ordinary people can make a difference. Empowering citizens can transform the India drug safety system. Always buy medicines from licensed and reputable pharmacies, and don’t ignore the batch and expiry details. Trusted outlets are more likely to participate in recalls and offer valid batch details. Before giving a new drug to your child, check official government lists or the Pharmacovigilance Programme of India (PvPI) and Adverse Drug Reactions Monitoring System (ADRMS) portals to see if any recalls or warnings exist. If something feels off – even a mild side effect – report it using easy online tools. Empowered citizens keep pressure on authorities and companies alike. Public engagement is a vital antidote to complacency.
The more we talk openly about medicine safety, the stronger our pressure on regulators and companies gets. Let’s not forget: every parent has the right to question medicine quality and demand real accountability. The louder we speak, the less likely a tragedy will go unnoticed. In this regard, sharing warning signs with others in our neighbourhood, in WhatsApp groups, and even with local doctors can catch problems faster. If more Indians demand up-to-date information and supply-chain accountability, government and industry will be forced to modernize rapidly. These small steps, multiplied nationwide, build a culture of vigilance and health equity.
Turning Tragedy into Accountability
Ultimately, the Coldrif tragedy shows that even now, India’s drug safety system has long-standing weak points that cost lives. Each time a family suffers, it should push us all to demand more – better enforcement, better transparency, and much faster action. If we don’t push, nothing changes, and those numbers on the chart are more than stats – they’re real children, real parents, real pain. Public health and trust are non-negotiable, and the need for urgent, real reform is plain for all to see.
As Justice D.Y. Chandrachud said, “When the system fails our children, reform is the only answer. Complacency is not an option.” If each of us calls for stricter oversight and supports public reporting and reform, there’s hope for a truly safe India drug safety system. This must be our rallying call for robust oversight, accountability, and compassion.
Are you prepared to demand urgent, transparent reforms, and help shape a safer drug safety system for India that every family deserves?
#IndiaDrugSafety #ColdrifTragedy #DrugRegulationIndia #PharmaAccountability #SafeMedicines

Cough syrups have been black marked since 1950s. It used to contain alcohol and later they prohibited alcohol and replaced it with glycerol.
The problem per se is not with the cough syrup rather where and how it is made.
In Mumbai there is a famous slum called Ulhasnagar. Many medicines are made here by throwing all rules and regulations to the wind. It is a multimillion worth cottage industry.
To top it all they affix the stamp “made in USA”. Here USA is Ulhasnagar Slum Association.
The drug controllers know this, and the govt. knows it.
they cannot move even their little finger because it is owned and patronized by many politicians.
Before we take any medicine say an “OUR FATHER”.